The following Course is the ETHICS in RESEARCH COURSE as it was offered by the National Institute of Health (NIH). This course is a prerequisite to the FHCi HP Certification process as we want to be sure that all HP Supervisors have a basic understanding of this topic for yourself, FHCi, our HP families, the public, and other health care professionals you may be communicating with. FHCi engages in continued research of Homeopathic Nosodes and infectious disease for infants, children and adults. 
This course contains the original slides from the National Institute Of Health (NIH) Ethics in Research Course. FHCi has borrowed the course content without permission.
This course provides a 4 hour certificate that is not ACHENA approved. NASH or Other professional trade organizations accept non-ACHENA continuing education. A certificate of completion in Ethics in Research will be e-mailed to you upon completing the Course Proficiency Questions.
Register here ($35)
Part 1. FHCi Ethics and Legal Issues for HPx
This session is an FHCi presentation covering the ethics and legal issues regarding working in the field of infectious disease and the use of homeoprophylaxis (2hrs Prerecorded Video)
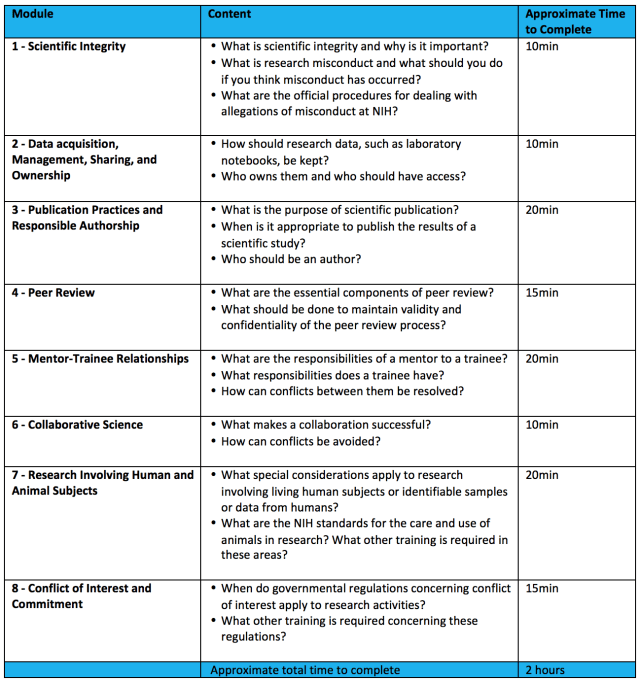
Part 2. Modules: (2hrs self-paced PowerPoints)
Outline of Modules in the Course: